Abdominal aortic aneurysms: Improved diagnostic confidence, patient experience
By Philips ∙ Feb 01, 2020 ∙ 3 min read
Abdominal aortic aneurysms (AAAs) cause more than 175,000 deaths globally every year, with an 80% mortality rate if ruptured.1 Routine surveillance is important, yet imaging modalities in the current standard of care are associated with significant drawbacks. Philips AAA Model overcomes these drawbacks, offering increased diagnostic confidence and an improved patient experience.
At-a-glance

Standard of care for AAAs
Typically, AAAs are identified incidentally during abdominal imaging exams, but in some cases can remain undetected until rupture. The current standard of care for AAAs requires several imaging modalities including 2D ultrasound and CTA, but each of these methods has its drawbacks: inter-operator variability with 2D ultrasound and patient exposure to high levels of radiation and nephrotoxic contrast agents with CTA.
Philips AAA Model is a software application that detects, segments and quantifies 3D ultrasound data for use in surveillance of native and post-endovascular aneurysm repair (EVAR) AAAs.
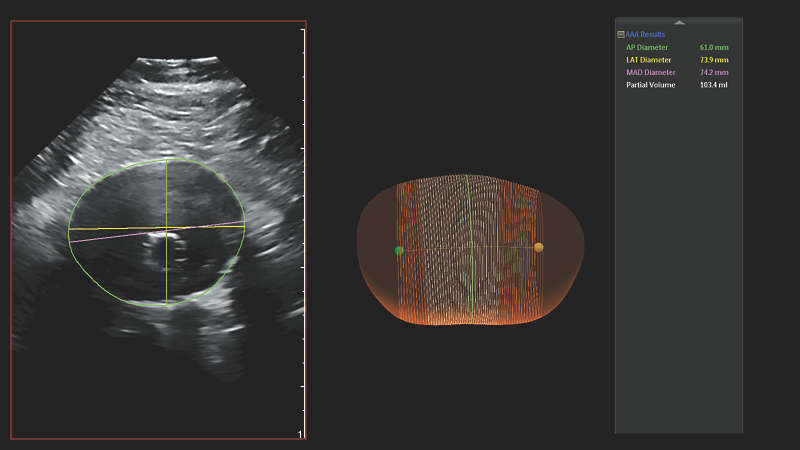
Philips AAA Model provides key measurements, including the maximum anterior-to-posterior (AP) diameter and partial volume of the aneurysm, while also indicating the centerline of the aneurysm. Philips AAA Model provides clinicians with the necessary diagnostic information without the drawbacks of 2D ultrasound and computed tomography angiography (CTA) in the current standard of care.
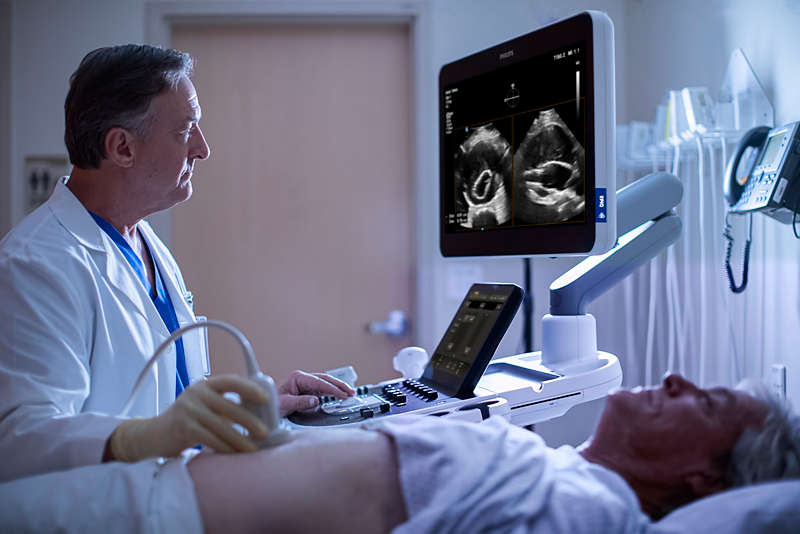
Improved patient experience
Philips AAA Model improves the patient experience by eliminating exposure to high levels of radiation and nephrotoxic contrast agents while still providing clinicians with the necessary diagnostic information.
Comparison of radiation exposure among common medical imaging procedures4
| Procedure | Approximate effective radiation dose | Comparable time period of natural background radiation |
| Ultrasound | 0 mSv | 0 years |
| Computed tomography (CT): abdomen and pelvis | 10 mSv | 3 years |
| X-ray: chest | 0.1 mSv | 10 days |
| X-ray: dental | 0.005 mSv | 1 day |
| Mammography | 0.4 mSv | 7 weeks |
It has been shown that 3D ultrasound can be used to estimate the diameter and volume of an AAA with acceptable reproducibility and an improved agreement with CT.2 3D ultrasound also correlates significantly better to 3D CT than the currently used method of 2D ultrasound when assessing maximum diameter of the residual sac after EVAR, with clinically acceptable reproducibility.3
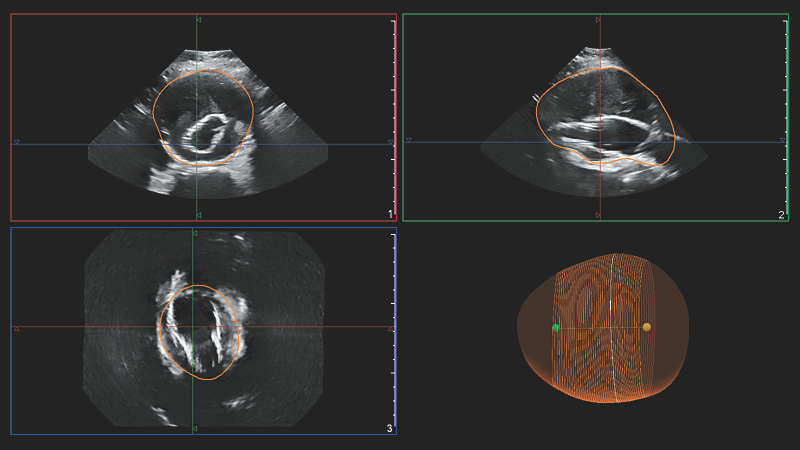
Increased diagnostic confidence
For surveillance of a native AAA by measuring the maximum AP diameter of the aneurysm, it has been shown that a 3D ultrasound exam can be used with inter-operator reproducibility superior to that of a 2D ultrasound exam.5 The range of inter-operator variability for 3D ultrasound was shown to be less than that of 2D ultrasound.
The maximal diameter of an AAA is ideally measured perpendicular to the centerline, a methodology that so far has only been feasible with 3D CT and magnetic resonance angiography (MRA).3 Philips AAA Model provides the centerline of the AAA throughout the volume of the 3D acquisition, making 3D ultrasound now also feasible with this methodology.
Philips AAA Model also provides the partial volume of the aneurysm. This is beneficial as it has been observed that more than one-third of small AAAs considered to be stable based on diameter alone were actually growing in volume.6 This suggests that volume measurements have the potential to supplant diameter as the most important single parameter in the diagnosis and surveillance of AAAs.6
The low cost of ultrasound, combined with the absence of both radiation exposure and administration of nephrotoxic contrast agents to the patient, has made it the preferred imaging modality for aneurysm screening and surveillance.7
Compatible ultrasound technologies
Philips AAA Model seamlessly integrates innovative software and leading Philips ultrasound technologies, including the Philips X6-1 xMatrix transducer and the Philips EPIQ Elite premium ultrasound system, into a single solution.
Clinical article
Increased diagnostic confidence and improved patient experience
Subscribe to our email updates
Footnotes
[1] Howard DP, Banerjee A, Fairhead JF, et al. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. British Journal of Surgery. 2015;102(8):907-915. doi:10.1002/bjs.9838. www.ncbi.nlm.nih.gov/pmc/articles/PMC4687424 [2] Bredahl K, et al. Three-dimensional ultrasound evaluation of small asymptomatic abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 2015;49(3):289-296. www.ejves.com/article/S1078-5884(14)00699-6/abstract [3] Bredahl K, Taudorf M, Long A, Lönn L, Rouet L, Ardon R, Sillesen H, Eiberg JP. Three-dimensional ultrasound improves the accuracy of diameter measurement of the residual sac in EVAR patients. European Journal of Vascular and Endovascular Surgery. 2013;46(5):525-532. www.sciencedirect.com/science/article/pii/S1078588413005753 [4] Radiological Society of North America, Inc. Radiation dose chart for physicians: radiation dose to adults from common imaging examinations. 2018. www.acr.org/-/media/ACR/Files/Radiology-Safety/Radiation-Safety/Dose-Reference-Card.pdf?la=en [5] Ghulam QM, et al. Clinical validation of three-dimensional ultrasound for abdominal aortic aneurysm. Journal of Vascular Surgery. 2019. In Press. www.jvascsurg.org/article/S0741-5214(19)31126-7/abstract [6] Ghulam QM, et al. Follow-up of small abdominal aortic aneurysms using three-dimensional ultrasound: volume versus diameter. European Journal of Vascular and Endovascular Surgery. 2017;53(3):e17. www.ejves.com/article/S1078-5884(16)30641-4/abstract [7] Chaikof EL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery. 2018;67(1):2-77.e2. www.jvascsurg.org/article/S0741-5214(17)32369-8/abstract


