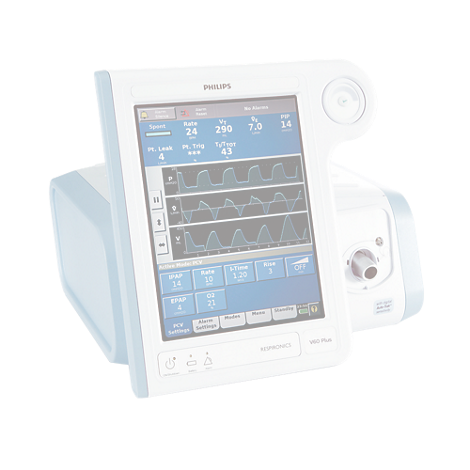
V60 Plus Ventilator
High flow therapy is not for use on the V60/V60 Plus ventilators in the USA after November 7, 2023.
This product is no longer available
Find similar productsThe Philips Respironics V60/V60 Plus ventilators provide noninvasive (NIV) and invasive mechanical ventilation (IMV) for spontaneously breathing adult and pediatric patients (weighing >20 kg/44 lbs). High Flow Therapy* was provided for use in accordance with FDA’s guidance, “Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency,” Section IV Policy for Modifications to FDA-Cleared Devices, issued March 2020. The V60 Plus ventilator and high flow therapy upgrade, are not FDA cleared.